|
|
>>
New User Register 

Login Member:
|
|
|
|
| This educational physics (atomic) animation with audio shows Bohr's creation of the atomic model in a systematic manner. It starts from the mystery of spectral lines and ends with the explanation of hydrogen spectra, an animated glossary and a quiz on Bohr's model. This animated 1.5 hour Bohr's atomic model covers in detail the endeavor of the scientists to see the unseen and is meant for high school and college atomic physics classes.
|
This animation has audio.
Category : Physics Atomic
Type : Animation with sound
Animation Type : Advanced
Total animation length: 1 hr 30 minutes
Refer details section for information.
|
|
|
|
|
The animation answers the questions like
What is the radius of orbits in which electrons did not radiate?
What was the energy possessed by the electron in these orbits? |
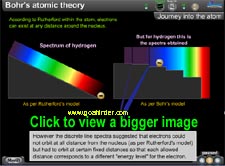 |
 |
|
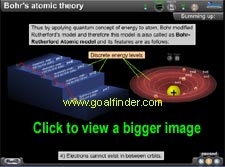
The physics animation exhibits the path-breaking concepts of Bohr through comparison between orbital, energy well and energy level model and calculations for speed, energy, radius and their correlation. |
|
 |
Bohr's postulates, explanation of hydrogen spectra and its line series are elaborated also the shortcomings of the model are completely covered. Our animated mascot, professor Stanley quizzes you on the concepts. An animated glossary clarifies the terms like Rydberg's constant, ionization energy, quantum well etc. |
|
This Bohr's atomic theory animation is directed towards high school / university students and teachers. It contains animation supported by audio and is directed towards unfolding the efforts of Bohr to apply quantum principles to Rutherford's mechanistic planetary model. Derivations, formulas, hints, explanations and glossary are provided for better grasp of the concepts. A quiz checks the understanding. Topics covered are:
Journey into the atom - Bohr strives to save Rutherford's model :
Mystery of missing spectral lines from emission and absorption spectra, Rutherford's model, Bohr's postulates.
Visual comparison of Bohr's and Rutherford's model :
Columbic forces balance mechanical rotation, understanding spectra, continuous and discrete spectrum, why did the electrons not fall into the nucleus? Ramp and steps and unequal steps analogy.
Which orbits were considered to be stationary by Bohr? :
Quantifying of angular momentum
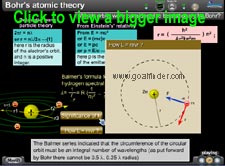
Derivation of L= nh/2pi :
DeBroglie's equation
How did he bring the above restriction on the circular orbits or stationary states? Planck's constant 'h' and Einstein's equation E = hf . Calculation of photon's wavelength. stationary states
Balmer's formula
Quantum theory formulated by Max Planck and established by Einstein : A short explanation
Significance of "h"
How L = mvr ?
More on quantum : Quantum world and laws are different from real world and laws.
Concept and Derivation of radius of hydrogen atom : Electrons can only exist at quantized distance from hydrogen nucleus.
Determining the total energy possessed by an electron
Calculation of orbital speed
Calculation of energy
Correlation between n, energy level and orbital radius
Bohr’s postulates
Explanation of hydrogen spectrum in detail : Why spectral lines are produced? Correlation between orbital and energy level model .Understanding absorption and emission spectra of hydrogen. Intensity of spectral line.
Explanation of Lyman, Balmer, Paschen, Pfund, Brackett, Humphreys spectral line series
What if the atom is subjected to continuous intake of energy?
Summing up
Shortcomings of Bohr's Model : Zeeman, Stark effect, discrete energy levels, larger atoms, position momentum.
References : See below
Quiz
Reference cover the terms in details:
Ångström
Electron Volt
Formula for Energy,
Radius and
Wavelength
Hydrogen Energy
Levels
Ionization Energy
Kilo joules per mole
Nanometer
Quantum Well
Rydberg's constant
for Hydrogen
Series derived from
Rydberg's formula
|
|
|
|
|
| |
 very good by Gianluca Lapini
very good by Gianluca Lapini |
| |
|
|
|
|
|

