
Why is it difficult to cut a
fruit using a blunt knife? Why drawing pins have flattened
heads? On blowing why does a rubber balloon swells? The
answers to all these questions are related to the effect
of a force applied on a surface. This effect is termed as
pressure.
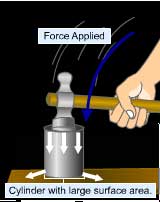
Pressure is the force acting on an unit area of the surface
in a direction perpendicular to it.
Pressure =
Perpendicular contact force (Newton N)
--------------------------------------------
surface area of contact (square meter or m2)
The S.I unit of pressure is Pascal( N/m2 )
When the force of one Newton acts on an area of one square
meter, the pressure on that surface is said to be one Pascal.
Thus
1.Pressure is directly proportional to force
on same contact area.
2. Pressure is inversely proportional to surface area
i.e. When same force is applied on different areas, greater
is the pressure for smaller contact area while smaller is
the pressure for larger contact area.
Similarly when different forces are applied on the same
surface area, greater the magnitude of force greater is
the pressure.
Though force is a vector quantity pressure is a scalar
quantity since it is independent of direction.

All things on earth are constantly hit by large number
of air molecules present on the surface of earth (the envelope
of air extends up to 320 miles).
These molecules though invisible have weight and cumulatively
exert pressure on all things on earth. The pressure exerted
by these air molecules is called as Atmospheric pressure.
The atmospheric pressure at a place is the weight of a column
of air over a unit area of the earth's surface at that place.
Atmospheric pressure decreases with increase in altitude.
This is because the number of air molecules i.e. air density
decreases at higher altitudes.
At higher altitudes due to weak gravitational force, the
cumulative force exerted by the air molecules on an object
decreases and hence atmospheric pressure decreases.

When a rubber balloon is blown into, it swells in all directions
and takes a rounded shape. This happens because as one blows
into a balloon, air molecules are injected into it. These
molecules while moving randomly around the space collide
with the skin of the balloon and thus exert pressure in
all directions. This pressure acts perpendicular to the
surface and helps the balloon to swell and take a round
shape. |

