|
|
>>
New User Register 

Login Member:
|
|
|
|
| This physics animation explains in detail the esoteric concept of internal energy and its variance with temperature and work. The concept of internal energy is presented through one-hour animation. This animation is meant for high school and college physics classes.
|
Category : Physics
Type : Animation
Animation Type : Advanced
Total animation length: 60 minutes
- What is internal energy?
- Internal energy in matter
- Internal energy of a classical ideal gas
- Can internal energy be changed?
- Change in internal energy
-- Case1: By doing work
-- Case 2: By heating (or due to temperature difference)
- How are internal energy and temperature related?
- Potential energy
- Potential energy at atomic level
- Kinetic energy
- Kinetic energy at molecular level
|
|
|
|
|
Detailed animation shows internal energy, work and heat are inter-related |
|
|
|
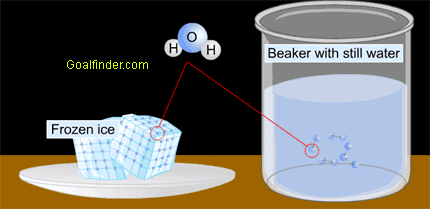
In-depth animated description of matter at molecular level to understand the internal energy of ice and water. |
|
|
 |
|
Animated experiments and analogies of molecular degrees of freedom with real life examples explains concept in a lucid manner. |
|
Internal energy of an object can be changed by the following methods:
1. It increases if energy is added to the system i.e. by heating or by doing work on the system.
2. It decreases if energy is removed from the system or work is done by the system.
i.e. Thus heat and work changes the internal energy of an object.
This science animation answers
the following questions in detail:
- What is internal energy?
Is internal energy a macroscopic or microscopic entity?
- Internal energy in matter
IE for each of the three states of matter on the basis of molecular motion and forces of attraction between them, and for polyatomic and diatomic gases using degrees of freedom of molecules.
- Internal energy of a classical ideal gas
IE of ideal gas and equipartion law
- Can internal energy be changed?
By using thermodynamic experimental processes proved that IE depends on the state of the system not on method of energy transfer and its rate.
-- Case1: By doing work
-- Case 2: By heating (or due to temperature difference), use of cartoons and examples in the animation eases the learning process. Explanation at molecular level and case by case approach for system and surroundings. Introduction to "First law of thermodynamics".
- How are internal energy and temperature related?
Using interactivity, the relationship between kinetic energy, IE, temperature is brought out in an easy to understand manner. Question answered : is temperature a form of energy?
- Potential energy
The concept of potential energy due to gravity , charges, bonding explained with everyday examples
- Potential energy at atomic level
- Kinetic energy
Kinetic energy explanation and calculation
- Kinetic energy at molecular level
Kinetic energy for basis on linear, rotational and vibration motion of molecules
- Explanatory Notes :
--- What determines the temperature of a single body
--- Can heat flow be reversed - from colder to hotter region
---
If two objects cannot be placed together, how do we determine whether they are in thermal equilibrium?
--- Why warm air becomes cooler as it rises up?
|
|
|
|
|
| |
|
|
|
|
|

