|
|
>>
New User Register 

Login Member:
|
|
|
|
| This 25-minute physics animation shows what is happening at molecular level and where does the energy absorbed go during phase change. Explanation includes effect of phase change on potential, kinetic energy and bond length between molecules. It is meant for high school and college science students.
|
Category : Physics
Type : Animation
Animation Type : Advanced
Total animation length: 25 minutes
- Refer Details section for content of the animation or else watch preview.
|
|
|
|
|
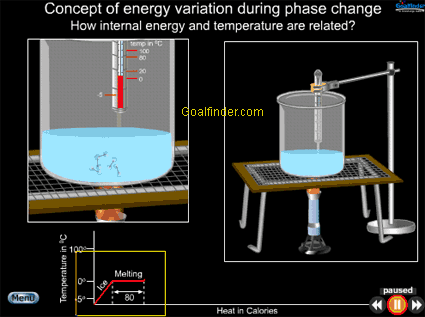
Animation shows macroscopic and microscopic view of phase change of ice simultaneously, at macroscopic level we see temperature increasing due to energy and microscopically we see increasing vibrations of molecules due to KE. |
|
|
|
Animated explanation of how a phase change involving change of energy of a substance, also affects its internal energy (IE). |
|
|
|
Co-relation between energy and temperature , why so much heat is required to convert water to vapor at 100 deg C.
|
|
This science animation explains the following in detail:
-
Change in energy of water from -5 OC to 0 OC , 0 OC to 100 OC and at 100 OC
- How are internal energy and temperature related?
- Potential, Kinetic energy and bond length between molecules.
- What are melting and boiling points?
- Where does the heat supplied to ice/water at these points go?
- Why does the average KE remain constant at 0OC?
- What is latent heat of fusion?
- Why does internal energy increase at melting / boiling point
- Why does it take 5 times the energy to convert water to vapor as compared to that used in heating it from 0O C to 100O C?
- At 100 OC besides breaking the bonds, what else is heat used for?
- Why does the PE reach a maximum at 100 OC and then suddenly become zero?
- What does the latent heat of vaporization convert into?
- Is temperature proportional to internal energy?
|
|
|
|
|
| |
 Good by amit
Good by amit |
| |
|
|
|
|
|

